Abstract
Introduction
Acute Graft-Versus-Host Disease (aGVHD) is a serious complication of hematopoietic stem cell transplantation (HSCT) that is primarily driven by alloreactive T cells. CD6 is a costimulatory receptor primarily expressed on T cells that promotes synapse formation, T cell activation, and migration into tissues by engaging with its ligand, activated leukocyte cell adhesion molecule (ALCAM). CD6 is expressed on reconstituting T cells soon after HSCT (Rambaldi et al., 2019) and early studies have shown that ex-vivo depletion of CD6 + donor cells prior to HSCT decreases the incidence of aGVHD (Soiffer et al., 1992; Soiffer et al., 1998). The reduced levels of aGVHD were attributed to an increased prevalence of CD6 - T cells that were less alloreactive (Rasmussen et al., 1994). Therefore, modulating activity of the CD6-ALCAM pathway may ameliorate aGVHD. Itolizumab is a humanized anti-CD6 monoclonal antibody that was previously described to block the engagement of ALCAM, thereby inhibiting T cell activity and trafficking. Here, we elucidate a second and highly novel mechanism in which antibody-mediated loss of CD6 from the surface of T cells results in CD6 low T cells that are hyporesponsive to T cell stimulation.
Methods
To assess the molecular mechanisms for itolizumab-induced loss of cell surface CD6, peripheral blood mononuclear cells (PBMCs) were exposed to different conditions including treatment with selected protease inhibitors, a membrane inhibitor, or Fc receptor blocking antibodies in the presence of itolizumab. Furthermore, B cells, NK cells, and monocytes were isolated from PBMCs and mixed with T cells in combination with itolizumab to evaluate cell contact requirements for loss. Following treatment, cell surface levels of CD6 protein were assessed by flow cytometry using a non-competing anti-CD6 detection antibody while analysis of full length CD6 was detected by western blot from total protein lysate. The soluble form of CD6 (sCD6) was analyzed by electrochemiluminescence and immunoprecipitation from supernatant of treated cells.
Results
Following treatment with itolizumab, the levels of cell surface CD6 was reduced as assessed by flow cytometry and western blot. Concurrent with a loss of cell surface CD6, levels of sCD6 in the cell supernatant increased, suggesting CD6 is primarily cleaved from the cell surface rather than internalized. Characterization of the cleaved sCD6 product by western blot revealed a 30KDa product. Itolizumab-induced changes in both levels of cell surface CD6 and sCD6 was abrogated by both 4-benzenesulfonyl fluoride hydrochloride (AEBSF), a serine protease inhibitor and cytochalasin D, an inhibitor of actin polymerization that prevents movement within the cell membrane and blocks endocytic trafficking. This suggests that a membrane-bound serine protease is responsible for cleavage.
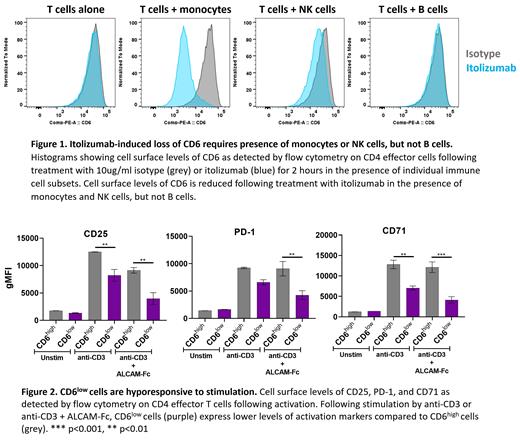
Itolizumab-induced CD6 cleavage did not occur with T cells alone and required the presence of other immune cells including monocytes and NK cells, but not B cells (Figure 1). In addition, when monocytes and T cells were separated by a membrane, cleavage of CD6 was not observed in the presence of itolizumab, indicating that cell-to-cell contact is required. Furthermore, blocking antibodies indicated that functional Fc receptors, especially FcγRIA, were required, suggesting that binding of itolizumab to both an Fc receptor on monocytes and CD6 on T cells predicated the cleavage event. The resulting CD6 low T cells were hyporesponsive to T cell stimulation, even in the absence of itolizumab, as indicated by reduced expression of PD-1, CD71 and CD25 as well as inflammatory cytokines (Figure 2). Inhibition was observed for both naïve and effector/memory T cell subsets.
Conclusions
Our results reveal a novel mechanism of antigenic modulation by itolizumab in which CD6 is cleaved from the T cell surface and released in a soluble form. Cleavage of CD6 occurs via a membrane-bound serine protease and appears dependent upon the engagement of itolizumab with Fc receptor(s) present on monocytes and NK cells. The loss of cell surface CD6 results in T cells with reduced responses to TCR-mediated stimulation. As such, treatment of aGVHD patients with itolizumab may reduce the alloreactivity of donor T cells, ameliorating disease symptoms and improving the clinical outcomes in these patients.
Chu: Equillium: Current Employment. Marrocco: Equillium, Inc.: Current Employment. Tiet: Equillium, Inc.: Current Employment. Ampudia: Equillium, Inc.: Current Employment. Connelly: Equillium: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees. Ng: Equillium: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal